- Nutrients, foods, and diets
- Dietary adequacy and nutritional requirements
- Diet variation, quantification, and misreporting
- Food composition data and databases
- Data processing
- Introduction to subjective measures
- Estimated food diaries
- Weighed food diaries
- 24-hour dietary recalls
- Food frequency questionnaires
- Diet checklists
- Diet histories
- Technology-assisted dietary assessment
Nutritional biomarkers
Nutritional biomarkers provide objective information on dietary exposure. Objective assessment is highly important to circumvent the fundamental limitation of measurement error in self-reported subjective assessment of dietary exposure [3,14,22].
Among prospective cohort studies assessing incident non-communicable diseases, EPIC-Norfolk is the largest cohort that has measured habitual dietary intake with subjective instruments and also nutritional biomarkers [9]. In one analysis, the investigators compared the association between habitual fruit and vegetable consumption and incident type 2 diabetes with that between plasma vitamin C as a biomarker of fruit and vegetable consumption and type 2 diabetes.
As seen in Figure D.17.1, there was a stronger inverse association when plasma vitamin C biomarker was examined, compared with self-reported fruit and vegetable intake from a food frequency questionnaire, across fifths (quintiles) of their distributions. This indicates a proof of principle that a nutritional biomarker can provide a method with less error than the subjective instrument to examine associations between dietary factors and disease.
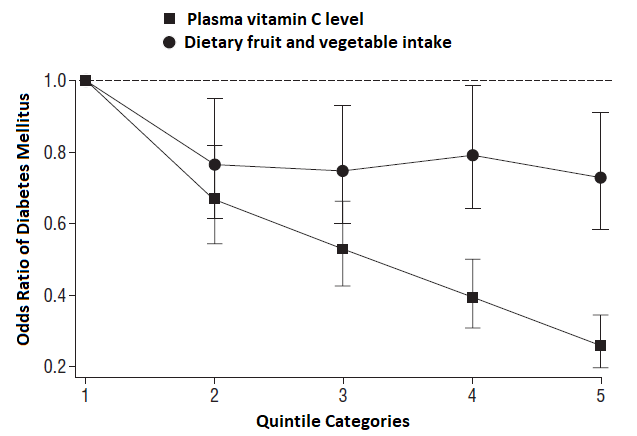
Figure D.17.1 Odds ratios for type 2 diabetes according to quintile of self-reported fruit and vegetable intake estimated from food frequency questionnaires [black circles] and plasma vitamin c concentration [black squares]. Analyses are adjusted
for age, sex, family history of diabetes, physical activity, smoking status, social class, education level, vitamin supplement use, body mass index and waist circumference.
Source: EPIC-Norfolk Study – adapted from [9].
Although many studies demonstrate utility of nutritional biomarkers, they are highly diverse in terms of applications, strengths, and limitations. Dietary dimensions assessed with nutritional biomarkers are shown in Table D.17.1. These vary depending upon the category of biomarker, as described below.
Definition of nutritional biomarkers
In the field, many definitions are proposed. In general terms, a nutritional biomarker can be defined as “any biological specimen that is an indicator of nutritional status with respect to intake or metabolism of dietary constituents. It can be biochemical, functional, or clinical index of status of an essential nutrient or another dietary constituent” [14].
Categories of nutritional biomarkers
There is more than one scheme used to categorise nutritional biomarkers. Biomarkers can be grouped according to what they assess [14]. A single biomarker may be included in one or more of the following categories:
- Biomarkers of dietary exposure assess dietary intake of different nutrients, non-nutritive components, foods, food groups, or dietary patterns.
- Biomarkers of nutritional status assess not only intake but also metabolism of the nutrient(s) and possibly the effects from disease processes. Potentially these may not reflect the nutritional status of a single nutrient, but may indicate the interactions of several nutrients.
Another classification scheme distinguishes recovery, concentration, predictive and replacement biomarkers as the followings [6, 10, 13]. The classification is not mutually exclusive.
- Recovery biomarkers are based on the concept of metabolic balance between intake and excretion during a fixed period of time. Recovery biomarkers are directly associated with dietary intake and can be used to assess absolute intake (not only to rank intake). There are relatively few recovery biomarkers, including doubly labelled water, urinary nitrogen, and urinary potassium.
- Concentration biomarkers are correlated with dietary intake and used for ranking of individuals. They are not used to determine absolute intake because they are related to metabolism, personal characteristics (e.g. age, sex), and lifestyle factors (e.g. smoking, physical activity). Examples of concentration biomarkers include plasma vitamin C or plasma carotenoids.
- Predictive biomarkers, which do not completely reflect dietary intake, but can predict it to some extent, such as urinary sucrose and fructose. Similar to recovery biomarkers, these are sensitive, time dependent and demonstrate a dose-response with intake; the key distinction is that their overall recovery is lower [21].
- Replacement biomarkers, which serve as a proxy for intake when it is not possible to capture because information in nutrient databases is unsatisfactory or unavailable. Examples include sodium, phytoestrogens, polyphenols or aflatoxin.
Table D17.1 Dietary dimensions assessed by nutritional biomarkers.
| Dietary dimension | Possible to assess? |
|---|---|
| Energy and nutrient intake of total diet | Yes |
| Intake of specific nutrients or foods | Yes |
| Infrequently consumed foods | Yes |
| Dietary pattern | Yes |
| Habitual diet | Yes |
| Within-individual comparison | Yes |
| Between-individual comparison | Yes |
| Meal composition | No |
| Frequency of eating/meal occasions | No |
| Eating environment | No |
| Adult report of diet at a younger age | No |
Specimen collection
Nutritional biomarker methods rely upon biological specimen being collected from the participant. Examples of biological specimens include:
- Serum and plasma: Reflect the short term intake from a few days to one month.
- Erythrocytes: Reflect longer-term intake than serum and plasma, because of their half-life (approximately 120 days), but shorter than adipose tissue.
- Adipose tissue: Reflects long-term intake, most useful to assess exposure to fat-soluble vitamins and essential fatty acids.
- Urine: Reflects short-term intake. Samples collected can be 24-hour samples, overnight samples or spot samples. 24-hour samples are suitable for recovery biomarkers such as nitrogen, potassium and sodium or prediction biomarkers such as sucrose plus fructose. However, since they can be burdensome for participants, it is necessary to assess the completion of urinary sampling in a day. The compliance can be assessed with the use of para-aminobenzoic acid (PABA). PABA is assumed to undergo urinary excretion in 24 hours, so if PABA recovery is high (e.g. >85%), then the collected urine can be considered complete [2].
- Hair and nails: Reflect long-term intake. They are easy to collect and store, but it is possible that environmental contamination occurs (particularly for hair) [26, 27].
- Cheek cells: Simple to collect.
- Blood spot from fingertips: Simple to collect.
- Stool/faeces: Difficult to collect, higher burden for the participants.
Other bio-specimens may include leucocytes, cord blood, breast milk, saliva, sweat, and any biopsied tissue.
Timing of specimen collection
- Time of day: Biomarker levels can be influenced by the time of the day if measured in samples which reflect the short-term intake. One example is the diurnal variation that occurs with some biomarkers, so the timing is important to note. Ideally, time of the day should be standardised when the samples are collected. By contrast, measures in samples that reflect longer-term intake, such as nails, hair, and adipocytes, for example, do not fluctuate within a day.
- At fasting or non-fasting state: Similar to time of day, the physiological state of an individual should be considered. Some fat-soluble markers (e.g. carotenoids) are partly present in lipoprotein cholesterols, and thus measures from a postprandial blood sample need to be interpreted carefully.
- Seasonal variation: This can exist for two reasons at least. First, seasons influence the availability of food sources of biomarkers (e.g. plasma lycopene reflecting tomato consumption). Second, seasons influence the variability of a biomarker (e.g. 25-hydroxy vitamin D levels are higher during summer because of higher sun exposure).
Storage
- It is advisable to store biological samples in different aliquots rather than a single tube, as repeated freeze-thaw cycles can affect the biomarker.
- Most samples should be frozen at a very low temperature (-80°C) to avoid degradation, although it is ideal to freeze at even a lower temperature using liquid nitrogen.
- Measurement of biomarkers in blood cells requires isolation of these samples from the blood and separate freezing.
- Good labelling and traceability of frozen samples are essential.
- Use of different anti-coagulants in collection tubes may affect biomarkers.
- Different samples are characterised by different tolerance over long periods of storage [22]. Some types of nutrients and chemicals and types of samples are more prone to degradation and contamination. Acidity, temperature, exposure to light and other factors need to be controlled, depending on target molecules to assay. Before collecting
bio-specimens, pre-specification of molecules to assay and a pilot study are thus warranted. Examples include:
- Vitamin C biomarker is known to be oxidized without storing with meta-phosphoric acid, which stabilises it.
- Trace minerals assays are known to be contaminated with metals ubiquitously present in sample tubes and any other materials [17].
- Several vitamins (e.g. riboflavin, vitamin K) are known to be photosensitive.
Assessment of nutritional intake and status
Some nutrient intakes are particularly difficult to assess by subjective reporting. An example is dietary sodium, which is present in many manufactured food products and to different degrees even for the same food item. It is also added during cooking or at the table, often inconsistently and without measuring the amount added. Due to the high risk of measurement error associated with subjective reporting, objective measurement of urinary sodium is used to assess sodium intake in epidemiological studies.
The UK implemented a population-based intervention to gradually reduce sodium content in foods over time. Assessment of 24-h urinary sodium before, during, and after the intervention confirmed reduction of salt intake over time [18].
Nutritional biomarkers are used to identify nutritional adequacy. For example, iron deficiency is determined by using a combination of serum ferritin and transferrin receptors. For clinical diagnosis of deficiency diseases, confirmation is needed with a combination of other clinical examinations (e.g. symptoms).
Biomarkers of nutritional status are not necessarily nutrients. Examples include:
- Methylmalonic acid levels are elevated in a deficiency state of vitamin B12 and therefore serve as a biomarker of vitamin B12 status.
- Homocysteine levels are elevated in the absence of enzymes to metabolise it to cysteine or methionine. The enzymatic reactions require vitamins including vitamin B6, vitamin B12, and folic acid, and thus elevated homocysteine may indicate the lack of any of these nutrients.
- Many vitamins have co-enzymatic functions. Thus, enzymatic activity can reflect nutritional status and serve as nutritional biomarkers. For example, erythrocyte glutathione reductase uses riboflavin as a cofactor and thus its activity to catalyse oxidation of glutathione, measurable by fluorescence assay, can be a biomarker of riboflavin status.
Biomarkers for validation or calibration
Biomarker methods are not without errors and do not necessarily reflect a habitual diet unless repeatedly measured over time. Therefore, strictly speaking, a ‘validation’ study on the validity of a dietary assessment method is often argued to be called a ‘calibration’ study.
Biomarkers have often been used to examine validity of subjective methods of dietary assessment. Validity assessment of an error-prone instrument is often performed against a reference measure from another instrument (e.g. food-frequency questionnaires compared to weighed food diaries). Comparison between different instruments is informative, but both involve limitations of subjective methods. Use of objective biomarkers is thus important.
Use of recovery biomarkers is ideal, such as doubly labelled water or 24-hour urine collections. However, these are often expensive or inconvenient for participants [3]. Instead, concentration or prediction biomarkers are often used to evaluate the performance of a dietary assessment method. If a valid calibration equation is available, levels of concentration biomarkers and prediction biomarkers can be translated to estimates of absolute intakes. Each type of biomarkers has its own limitation (see below in section 5).
Examples of validation studies
The Observing Protein and Energy Nutrition (OPEN) Study was conducted in the United States from 1999-2000 and sought to assess the dietary measurement error of two self-reported instruments: the food-frequency questionnaire (FFQ) and two 24-hour recalls [19, 20]. Doubly labelled water, urinary nitrogen, and urinary sugars (fructose and sucrose) from two 24-hour urine samples were used as unbiased biomarkers of energy, protein, and sugar intakes in nearly 500 men and women. Key results were:
- Greater under-reporting of both energy and protein in both men and women by the FFQ compared to the average of two 24-hour recalls.
- Ability of estimating % of energy from protein and ranking individuals for protein intake was similar between FFQ and 24-hour recall, for which adjustment for energy intake was considered particularly important.
- This study also highlights the importance of the design of a validation study. All the data were collected for a short term period. By design, compared to FFQ, 24-hour recalls reflecting a 1-day diet should be more correlated with biomarker data reflecting a 1-day or short-term dietary exposure.
The EPIC-Norfolk Study assayed urinary measures of nitrogen, potassium, sodium, and plasma measures of vitamin C and polyunsaturated fatty acids. Then, the Study compared those with corresponding measures of dietary intakes based on a semi-quantitative FFQ and a 7-day diet diary [5, 23]. Key findings indicate that the degree of validity depends on the nutrient:
- 7-day diet diary performed consistently better than the FFQ for urinary measures and plasma vitamin C.
- 7-day diet diary and FFQ performed similarly for the assessment of polyunsaturated fatty acid intakes.
Laboratory analysis
Accurate assessment of dietary intake using biomarkers depends upon not only sample collection but also the analytical measurement of the biomarker.
Minimising measurement error
Efforts should be made to standardise the collection, storage and analysis of specimens, but this may not be possible in large multi-centre studies which take place over a long time period. Measurement error may result from [4]:
- Errors or omissions in compliance with specimen collection protocol
- Errors in sample storage
- Errors during specimen analysis
- Effects from reagents, instruments and interfering substances
- Variability in technician technique
- Use of different or contaminated reagents
- Failure to maintain standardisation of the instrument
One key step to minimise laboratory measurement errors is blinding of the characteristics of the specimens, such as disease status. Ideally all specimens should be analysed consecutively to reduce between-assay variation. If a matched case-control design is used, the samples from matched individuals (e.g. pairs) should be analysed in the same batch, and samples in a batch should be randomised. Quality control samples are available to ensure compatibility of results between batches or laboratories. It is also possible to test reliability by conducting blinded duplicate analyses of specimens [4].
A quality control program should [4]:
- Monitor clerical errors
- Provide detailed instructions on technique for different specimen types
- Monitor proper labelling and logging in/out of specimens
- Monitor labelling effects of reagents and materials
- Confirm absence of contaminants
- Ensure equipment is calibrated
- Calibrate study samples based on quality control sample measurements
Assessing measurement error
The coefficient of variation can be used to assess the reliability of laboratory analyses of the same sample, and this can be calculated both within and between runs.
Combination with subjective estimates
Dietary intakes can be estimated by combining biomarkers with subjectively reported data. These estimates should be more valid as one method can account partly for the disadvantages of the other method i.e. biomarkers account for dietary misreporting and self-reported methods account for errors associated with the metabolism of nutrients [15]. Thus, although concentration or prediction biomarkers themselves cannot indicate absolute levels of dietary intakes, they can be used to predict absolute intakes by combining with subjective estimates.
Key characteristics of nutritional biomarkers are summarised in Table D.17.2. All biomarkers have some limitations, and the degrees and types of limitations vary by biomarker. Some limitations may or may not be mitigated by the study design of sampling, assay, data processing, or data analyses for errors and confounding, and should be appraised appropriately.
Strengths
- Avoid biases related to recall, reactivity or social desirability
- Errors related to biomarkers are independent of those of self-reported dietary intake
- Several bio-specimens can be obtained easily without increasing participant burden or discomfort
Recovery biomarkers
- Reflection of absolute intake is useful in validation or calibration of self-reported estimates
Concentration or predictive biomarkers
- Can be used to rank individuals
Limitations
- Biomarker measurement is often more expensive than the use of subjective methods.
- Some biomarkers are difficult to obtain or may influence participation (e.g. tissue biopsies), posing ethical challenges and limiting the sample size.
- With a few exceptions, it is impossible to pinpoint the food source of a particular nutrient biomarker and therefore to make specific food/diet recommendations. For example, vitamin C biomarker may reflect consumption of whole fruits, fruits juices, potatoes, and dietary supplements. Although vitamin C as a biomarker is sensitive and specific, food sources and relevance to dietary guidelines could be diverse, depending on populations.
Here are some examples that a nutrient or food component is specific to a food source:
- Lycopene reflects tomato intake.
- The ratio of 13C to 12C, reflecting consumption of corns and canes (‘C4 plants’) [24]. Where common sugars derive from corns or canes, such as countries in American continents, the ratio was demonstrated to reflect self-reported intake of sugar-sweetened beverages.
- Alkylresorcinols, reflecting cereal intake, as the phytochemicals are concentrated in bran fraction of grains [12].
Recovery biomarkers
- Not frequently identified for dietary factors.
- Reflection of dietary intake over a relatively short time.
Concentration or predictive biomarkers
- Not reflective of absolute intake.
- Unclear how much they reflect true differences in intake vs physiological differences resulting in different biomarker concentrations.
Table D.17.2 Characteristics of nutritional biomarker methods.
| Characteristic | Comment |
|---|---|
| Number of participants | Any |
| Cost of development | High |
| Cost of use | High |
| Participant burden | Varies with sample type |
| Researcher burden of data collection | High |
| Researcher burden of coding and data analysis | High* |
| Risk of reactivity bias | No |
| Risk of recall bias | No |
| Risk of social desirability bias | No |
| Risk of observer bias | Minimised with blinding |
| Participant literacy required | No |
| Suitable for use in free living | Yes |
| Requires individual portion size estimation | No |
* Data coding and analysis here means processing of quantitative data from an assay apparatus.
Considerations relating to the use of nutritional biomarkers for dietary assessment are summarised by population in Table D.17.3.
Table D.17.3 Considerations relating to use of biomarkers for assessing diet in different population groups.
| Population | Comment |
|---|---|
| Pregnancy | Information on dietary supplements should be evaluated in detail. Cord blood samples would be non-invasive biological samples. |
| Infancy and lactation | Breast milk, a unique bio-sample. |
| Toddlers and young | Maturation may influence the utility of biomarkers (e.g. calcium, zinc) due to high turn-over rates of tissues. |
| Adolescents | Adolescent growth and menstruation in girls influence utility of specific biomarkers (e.g. calcium, iron). |
| Adults | In women, menstruation influences utility of specific biomarkers (e.g. iron). |
| Older Adults | Confounding by disease conditions and medications may occur, depending on the aim of the assessment. |
| Ethnic groups | Underlying genetic variability may influence utility of biomarkers (e.g. 25-hydroxy vitamin D [1]) which should be confirmed or used cautiously. |
| Other |
- The timing of specimen collection can result in measurement error due to within-individual biomarker variation. Variability within individuals can vary by the hour, by the year, or longer.
- Comparison of within- and between-individual variation should be considered for specific biomarkers. If the within-individual variation is higher, a larger sample size should be considered [4]. The between-individual variation can be minimised by standardising and using reliable collection and laboratory methods.
- When biomarkers are used as exposures in analyses of associations with disease outcomes, associations should be adjusted for potential confounders, as these will be an issue to the extent that the biomarkers reflect dietary intake.
Sensitivity and specificity of a biomarker
In clinical and epidemiological fields, ‘sensitivity’ and ‘specificity’ have been used in different ways, partly because a biomarker can be used to assess either nutritional intake or nutritional status. Here are some examples of ‘sensitivity’.
- ‘Sensitivity’ to assay or ‘analytical sensitivity’ would be high if a biomarker captures true intake, in a dose-response manner, even if the biomarker is present in low concentrations [8].
- ‘Sensitivity’ to nutrient status would be high if a biomarker identifies an abnormal nutritional status (e.g. deficiency) accurately. This use is consistent with the use in clinical epidemiology about accuracy of disease diagnosis.
Similarly, ‘specificity’ of a biomarker can be documented as the following:
- ‘Specificity’ or ‘analytical specificity’ would be high if a biomarker is not influenced by other factors [8].
- ‘Specificity’ to nutrient status would be high if a biomarker identifies normal nutritional status accurately.
Homocysteine is, for example, sensitive to folate intake but not specific, because it can vary by intakes of other B vitamins and amino acids. Thyroid stimulating hormone is sensitive to iodine deficiency, but not sensitive to a normal range of iodine intake [25].
Cut-off points of biomarkers
It would be ideal to have cut-off points to diagnose certain states of nutritional status or nutritional intake. However, cut-offs are rarely established. One reason is because any single biomarker has limitations and is often used jointly with other indicators, thus leaving definite cut-off points undetermined. Another reason is diversity of applications of a single biomarker to clinical diagnosis, screening, surveys, monitoring, and other complex objectives. See perspectives by Raghavan et al. [16] for more details.
Factors influencing sensitivity and specificity of nutritional biomarkers
Other factors might influence nutrient metabolism and may be potential confounders for an association between nutrient intake and nutrient biomarker. Many of the following considerations are based on clinical and biological knowledge. The impacts of such influences on biomarkers in population-based research remains unestablished. Examples include [10]:
Genetic Variability
- Genes that may affect dietary intake patterns, taste, attraction to or preference for specific foods or food types etc.
- Biological variation in nutrient absorption, digestion, metabolism, distribution, tissue turnover, and excretion.
- Epigenetic variation and gene-gene interactions.
- Gene variants are not necessarily an issue. Most gene variants influencing biomarkers are independent of dietary consumption. Therefore, unless a noticeable effect modification exists, gene variants do not confound a diet-biomarker association.
- Trained nurses/staff for the sample collection
- Materials for the sample collection
- Trained laboratory staff
- Laboratory equipment for the analyses
- Quality control documentation
Table D.17.4 Examples of nutritional biomarkers* and associated analytical methods.
| Nutrient | Test | Analytical method |
|---|---|---|
| Total energy | Doubly-labelled water | Mass spectrometry to assay isotope ratios (2H/1H and 18O/16O) |
| Protein | Urinary nitrogen | Kjeltec/Kjeldahl method |
| Omega-3 and -6 fatty acids | Fatty acid concentration in blood or tissue lipid compartment | Gas chromatography after transmethylation (Folch method) |
| Minerals | ||
| Potassium | Urinary potassium | Flame photometer |
| Sodium | Sodium potassium | Flame photometer |
| Calcium | Serum ionized calcium | Ion-specific electrodes |
| Phosphorus | Serum phosphorus | Colorimetry using molybdenum blue |
| Magnesium | Serum magnesium | Atomic absorption spectrometry |
| Serum ionized magnesium | Ion-specific electrodes | |
| Copper | Erythrocyte superoxide dismutase | Spectrophotometric assay or ELISA |
| Iodine | Urinary iodine | Acid digestion, followed by spectrophotometric assay using the Sandell-Kolthoff reaction |
| Iodine | Urinary iodine | Acid digestion, followed by spectrophotometric assay using the Sandell-Kolthoff reaction |
| Thyroid stimulating hormone | ELISA with dried blood spots or serum | |
| Iron | Serum ferritin | ELISA (in absence of infection) |
| Haemoglobin | Cyanmethemoglobin method. 'HemoCue' in field studies | |
| Serum transferrin receptor | ELISA | |
| Selenium | Plasma selenium | Atomic absorption spectrometry with Zeeman background correction or hydride generation atomic absorption spectrometry |
| Plasma glutathione peroxidase | ELISA: only useful if Se intakes are habitually low | |
| Zinc | Serum/plasma zinc | Flame atomic absorption spectrometry (in absence of infection) |
| Hair Zinc | Serum/plasma zinc | Measure by neutron activation analysis or atomic absorption spectrometry in children with low height percentiles and/or hypogeusia |
| Vitamins | ||
| Vitamin A | Liver retinol stores | HPLC |
| Modified relative dose response | Serum retinol and dehydroretinol via HPLC 4-6 h after oral dose of 3,4-didehydroretinol acetate (100 μg/kg) | |
| Vitamin D | Serum 25-hydroxyvitamin D | Separation of serum 25(OH)-D, followed by a competitive binding assay or radioimmunoassay, or tandem mass spectrometry |
| Vitamin E | Ratio of serum tocopherol to serum cholesterol | Reverse phase HPLC with a high sensitivity fluorescence detector |
| Vitamin K | Plasma vitamin K | HPLC, mass spectrometry |
| Thiamin | Erythrocyte activity of transketolase with and without added thiamin pyrophosphate | Semi-automated spectrophotometry using glyceraldehyde as an internal standard |
| Riboflavin | Erythrocyte activity of glutathione reductase with and without added prosthetic group flavin adenine dinucleotide | Enzyme-coupled kinetic assay where glutathione reductase activity is measured spectrophotometrically via oxidation of NADP to NADP+ |
| Niacin | NAD:NADP ratio in erythrocytes | HPLC |
| Pyridoxine | Plasma pyridoxal-5’-phosphate | Cation-exchange HPLC with fluorescence detection |
| Vitamin C | Serum or leucocyte ascorbic acid | HPLC with electrochemical detection. Should use preservative (metaphosphoric acid) |
| Folate | Erythrocyte and serum folate | Microbiological assay using L. casei |
| Serum homocysteine | Reversed-phase HPLC with fluorescence detection | |
| Vitamin B12 | Serum vitamin B12 | radioimmunoassay |
| Serum methylmalonic acid | Mass spectrometry | |
*Adapted from: [7, 8, 22].
Other molecules have been studied as markers of specific dietary intakes, such as:
- carotenoids, caffeine metabolites, alkylresorcinols, flavones, isoflavones, phytosterols, and phytochemicals
- mercury, cadmium, arsenic, and other ultra-trace
minerals
- trans fatty acids, conjugated linolenic acid, pentadecanoic acid, and heptadecanoic acid (fatty acids produced in ruminants)
- dioxins, heterocyclic amines, bisphenol A, and other contaminants
Statistical approaches to combine multiple methods including biomarker information and self-reported dietary data have been under rigorous methodological investigations over more than a decade [15]. While the methodology has been well conceptualised, there remain limitations in application, empirical evidence, and availability of software for wide use.
Some high-dimensional assays, including ‘omics’ technologies, have been used to identify new biomarkers, including:
- Metabolomics, measuring a large number of low-molecular-weight metabolites including essential amino acids and molecules not synthesized in the human body (e.g. trimethylamine N-oxide, TMAO, as a marker of fish/animal-based foods [11]).
- Epigenetics, including DNA methylation reflecting intakes of methyl donors (e.g. methionine) from diets and B vitamins (folate) playing key roles in the methylation cycle.
- Proteomics, potentially reflecting functional markers of nutritional intake and status.
- Aloia JF, African Americans, 25-hydroxyvitamin D, and osteoporosis: a paradox. Am J Clin Nutr. 2008;88:545S-550S
- Bingham S, Cummings JH, The use of 4-aminobenzoic acid as a marker to validate the completeness of 24 h urine collections in man. Clin Sci, 1983;64:629-35
- Bingham SA, Biomarkers in nutritional epidemiology. Public Health Nutr, 2003;5:821-7
- Blanck HM, Bowman BA, Cooper GR, Myers GL, Miller DT, Laboratory issues: use of nutritional biomarkers. J Nutr, .2003;133 Suppl 3:888S-894S
- Day N, McKeown N, Wong M, Welch A, Bingham S, Epidemiological assessment of diet: a comparison of a 7-day diary with a food frequency questionnaire using urinary markers of nitrogen, potassium and sodium. Int J Epidemiol, 2001;30:309-17
- Fraser GE, A search for truth in dietary epidemiology. Am J Clin Nutr, 2003;78:521S-525S
- Fu X, Peterson JW, Hdeib M, Booth SL, Grusak MA, Lichtenstein AH, Dolnikowski GG, Measurement of deuterium-labeled phylloquinone in plasma by high-performance liquid chromatography/mass spectrometry. Anal Chem, 2009;81:5421-5
- Gibson RS. Laboratory assessment. Principles of Nutritional Assessment 2ed. Oxford: Oxford University Press; 2005. p. 373-402.
- Harding AH, Wareham NJ, Bingham SA, Khaw K, Luben R, Welch A, Forouhi NG, Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer--Norfolk prospective study. Arch Intern Med. 2008;168:1493-9
- Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA, Biomarkers in nutritional epidemiology: applications, needs and new horizons. Hum Genet, 2009;125:507-25
- Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med, 2012;19:576-85
- Landberg R, Kamal-Eldin A, Andersson A, Vessby B, Aman P, Alkylresorcinols as biomarkers of whole-grain wheat and rye intake: plasma concentration and intake estimated from dietary records. Am J Clin Nutr, 2008;87:832-8
- Livingstone MB, Black AE, Markers of the validity of reported energy intake. J Nutr, 2003;133 Suppl 3:895S-920S
- Potischman N, Freudenheim JL, Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr. 2003;133:873S-874S
- Prentice RL, Tinker LF, Huang Y, Neuhouser ML, Calibration of self-reported dietary measures using biomarkers: an approach to enhancing nutritional epidemiology reliability. Curr Atherosc Rep, 2013;15:353
- Raghavan R, Ashour FS, Bailey R, A Review of Cutoffs for Nutritional Biomarkers. Adv Nutr, 2016;7:112-20
- Rodushkin I, Odman F, Assessment of the contamination from devices used for sampling and storage of whole blood and serum for element analysis. J Tr Element Med Biol, 2001;15:40-5
- Sadler K, Nicholson S, Steer T, Gill V, Bates B, Tipping S, et al. Assessment of Dietary Sodium Levels among Adults (aged 19–64) in England. 2011. Available from: http://transparency.dh.gov.uk/2012/06/21/sodium-levels-among-adults/
- Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol, 2003;158:1-13
- Tasevska N, Midthune D, Potischman N, Subar AF, Cross AJ, Bingham SA, Schatzkin A, Kipnis V, Use of the predictive sugars biomarker to evaluate self-reported total sugars intake in the Observing Protein and Energy Nutrition (OPEN) study. Cancer Epidemiol Biomark Prev, 2011;20:490-500
- Tasevska N, Runswick SA, McTaggart A, Bingham SA, Urinary sucrose and fructose as biomarkers for sugar consumption. Cancer Epidemiol Biomark Prev, 2005;14:1287-94
- Van Dam RM, Hunter D. Chapter 8. Biochemical Indicators of Dietary Intake . In: Willett W, editor. Nutritional Epidemiology. 3 ed. Oxford: Oxford University Press; 2012. p. 150-212.
- Welch AA, Bingham SA, Ive J, Friesen MD, Wareham NJ, Riboli E, Khaw KT, Dietary fish intake and plasma phospholipid n-3 polyunsaturated fatty acid concentrations in men and women in the European Prospective Investigation into Cancer-Norfolk United Kingdom cohort. Am J Clin Nutr, 2006;84:1330-9
- Yeung EH, Saudek CD, Jahren AH, Kao WH, Islas M, Kraft R, Coresh J, Anderson CA, Evaluation of a novel isotope biomarker for dietary consumption of sweets. Am J Epidemiol, 2010;172:1045-52
- Zimmermann MB, Andersson M, Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev, 2012;70:553-70
- Slotnick MJ, Nriagu JO, Validity of human nails as a biomarker of arsenic and selenium exposure: A review. Environ Res, 2005;102:125-39
- He K, Trace elements in nails as biomarkers in clinical research. Eur J Clin Invest, 2010;41:98-102
